40 open label study advantages and disadvantages
Advantages and disadvantages of clinical trials Possible advantages You may have access to new treatments, which may only be available as part of a clinical trial. There may be fewer side effects compared to the standard treatment. You may have more regular tests, which some people find reassuring. You will be supported by a research nurse - who you can contact about your treatment and symptoms. Drug Addiction: Advantages and Disadvantages | Free Essay Example Drug Addiction: Advantages and Disadvantages. A drug is a substance containing a chemical with the ability to change the normal biological processes and functions. It is used in medicine to correct or cure diseases and socially as a psychological stimulant to enhance pleasure. Drug addiction is a tendency of utilizing one or more ...
Crossover trials: what are they and what are their advantages and ... One can say that study participants serve as their own control. This leads to another advantage which is less study participants are required compared to a standard parallel randomized controlled trial (RCT). Reduction of sample size is consistent with the principle in medical research to use resources wisely.
Open label study advantages and disadvantages
(PDF) What is an open label trial? - ResearchGate The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to... Open-Label Extension Studies | SpringerLink Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised access to the drug from consumers and their physicians. Open-Label Trial | NIH - HIV.gov In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Skip to main content Get the latest public health information from CDC ... Double-Blind Study. Print. Print this term. Download Glossary. English Version PDF(3.13MB) Spanish Version PDF(3.16MB) CONNECT WITH US ...
Open label study advantages and disadvantages. Open-label extension studies: do they provide meaningful ... - PubMed Some increased confidence about incidence rates might result from the open-label extension study; however, as these studies are essentially uncontrolled and biased, the data are not of great value. Other benefits have been proposed to be gained from open-label extension studies. 16 Advantages and Disadvantages of a Double-Blind Study It is a disadvantage that can lead to a misinterpretation of the results being experienced in real-time. 3. It is not always possible to complete a double-blind study. There are times when a double-blind study is not possible. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the ... An open-label multicenter study in which patients with disease progression on two to six cycles of platinum therapy received single-agent cetuximab (initial dose 400 mg/m 2 followed by subsequent weekly doses of 250 mg/m 2 ) for 6 weeks (single-agent phase). Reducing bias in open-label trials where blinded outcome assessment is ... Many such trials are therefore open-label, where patients, clinicians, and care providers are aware of treatment allocations. For objective outcomes, such as all-cause mortality, unblinded assessment is unlikely to bias the trial results [ 5 ].
External and internal validity of open label or double‐blind trials in ... The simplified anticoagulant treatment in an open label trial may be less challenging for the patient and might increase patient retention. On the other hand, the inclusion of less compliant patients who would not be suitable for a double-blind trial may also increase drop-out rates in open-label trials. External and internal validity of open label or double-blind ... - PubMed In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-randomized treatment decisions and on reporting of outcomes. However, a blinded trial is not always feasible. Advantages and disadvantages of randomised control study design ... Publishable. Considered the gold standard: more publishable. Disadvantages of randomised control trial study design. Logistics: Power calculation might demand vast samples size, which require more resources from the investigators. Validity requires multiple sites, which will be difficult to manage. National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub- Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants or possible relief from symptoms of some disorders when a placebo is given. An open-label trial may still be randomized. Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. The Advantages and Disadvantages of Randomised Controlled Trials This chapter reviews the advantages and disadvantages of performing randomised controlled trials (RCTs). Observational studies may fail to include controls or may use an inappropriate comparison group whereas the RCT is, by definition, controlled with the best possible comparison group. This provides the main advantage of the randomised ...
Open-Label Trial - an overview | ScienceDirect Topics the difference of protective efficacy of ribavirin in humans and in hamsters may have several explanations: (1) the study in humans was not randomized and performed at posteriori, and grouping treated and nontreated patients may have introduced some bias; (2) the dose and mode of infection of niv used for the challenge or the virus replication in …
The Advantages & Disadvantages of Unblinded Sample Size Re ... - Statsols The advantages of unblinded sample size re-estimation clinical trials. Earlier Decisions. You inherit the Group Sequential Design which allows the researcher to stop early for efficacy or futility. This allows the ability to adjust the trial to the reality of what the effect size is telling you will happen in the trial. Reduced Potential Cost.
Effectiveness studies: advantages and disadvantages - PMC Advantages: Disadvantages: Placebo-controlled studies: Allow estimation of the assay sensitivity and thus internal validation of the study: Perhaps higher risk from "nontreatment" Allow better evaluation of the clinical relevance: Perhaps more limited generalisability of the results to the general population: Smaller sample size: Lower ...
Openstack Advantages And Disadvantages | ipl.org OpenStack advantages OpenStack is a free, open-source software platform for private clouds, usually used to deliver infrastructure as a service (IaaS). Consisting of interrelated projects that control pools of processing, storage, and networking resources throughout a datacenter, OpenStack lets users manage their cloud through a web-based ...
Review 2: advantages and disadvantages of mild cognitive impairment as ... This synthesis builds on initial mapping work (see Chapter 2) that followed systematic review principles, namely in undertaking a robust and transparent searching strategy and explicit data extraction. Our decision to use CIS6 recognises the characteristics of a complex body of literature that combines qualitative and quantitative studies and spans multiple disciplines. MCI is associated with ...
External and internal validity of open label or double-blind trials in ... disadvantages of open-label or double-blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-random-
Self-Manage Scleroderma | Lesson Patients have a chance to help others and improve patient care. Some disadvantages might be: New treatments or interventions under study are not always better than, or even as good as, standard care. Even if a new treatment has benefits, it may not work for everyone. Health insurance and managed care providers don't always cover clinical trials.
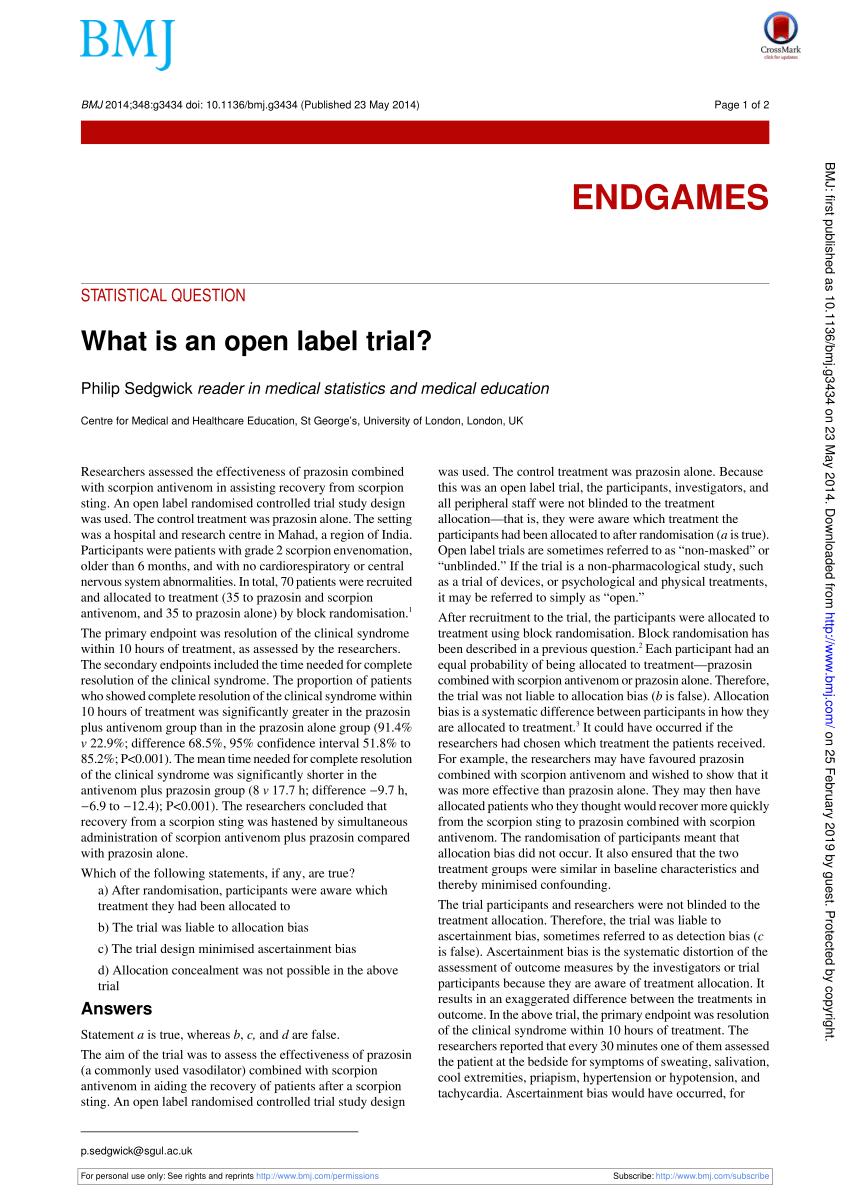
What is an open label trial? | The BMJ Because this was an open label trial, the participants, investigators, and all peripheral staff were not blinded to the treatment allocation—that is, they were aware which treatment the participants had been allocated to after randomisation ( a is true). Open label trials are sometimes referred to as "non-masked" or "unblinded."
Open-Label Trial - an overview | ScienceDirect Topics Serious adverse effects such as infections and hematopoietic suppression are rare. One advantage of MM, compared with other immunosuppressants, is a lesser carcinogenic tendency: a kidney transplant patient on MM had a lesser cancer incidence in the long-term follow-up. Lymphoma was reported as a rare complication.
External and internal validity of open label or double ... - ResearchGate ... 22 However, open-label trials offer the advantage of enhanced generalizability because the treatment quality in the warfarin control group may be more representative of routine clinical...
14 Advantages and Disadvantages of a Randomized Controlled Trial - Vittana This disadvantage can result in the loss of relevance for an idea because a practice can move away from the idea being studied by the time the trial is at a place where publication is possible.
Open-Label Trial | NIH - HIV.gov In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Skip to main content Get the latest public health information from CDC ... Double-Blind Study. Print. Print this term. Download Glossary. English Version PDF(3.13MB) Spanish Version PDF(3.16MB) CONNECT WITH US ...
Open-Label Extension Studies | SpringerLink Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised access to the drug from consumers and their physicians.
(PDF) What is an open label trial? - ResearchGate The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to...



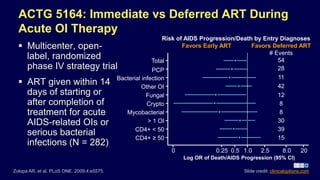
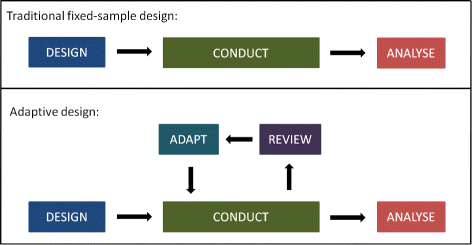

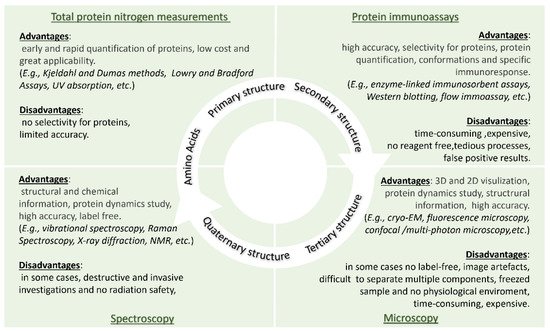
















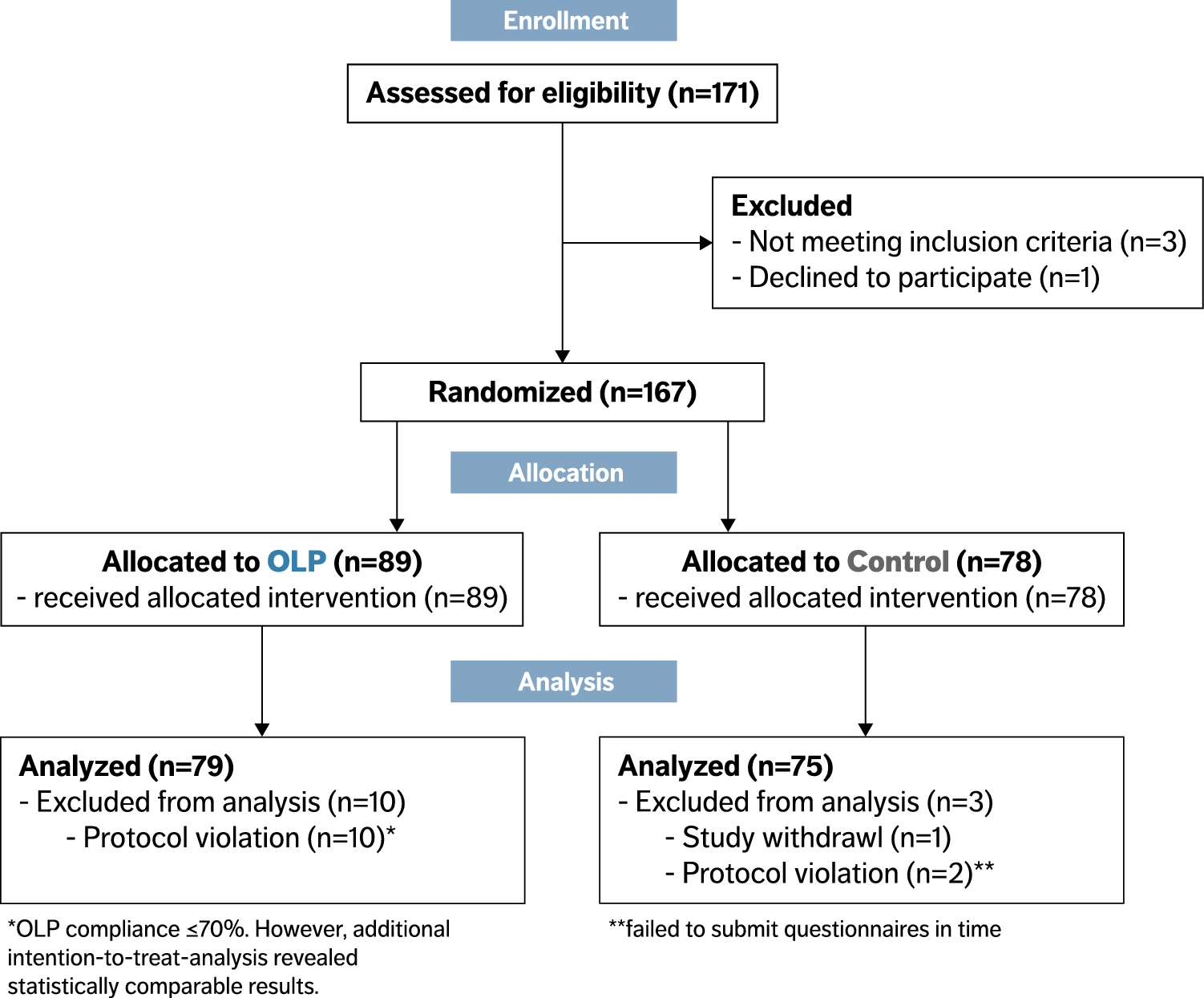

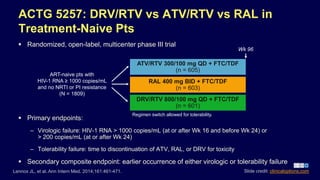


Post a Comment for "40 open label study advantages and disadvantages"