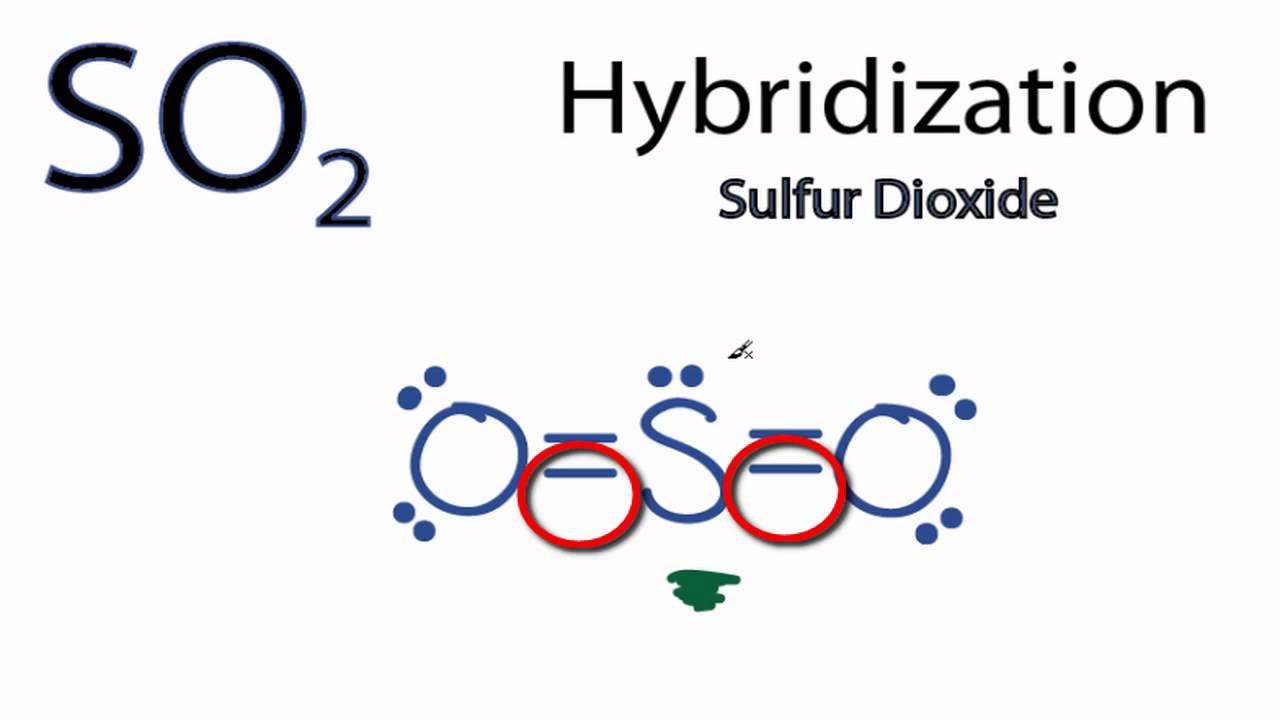
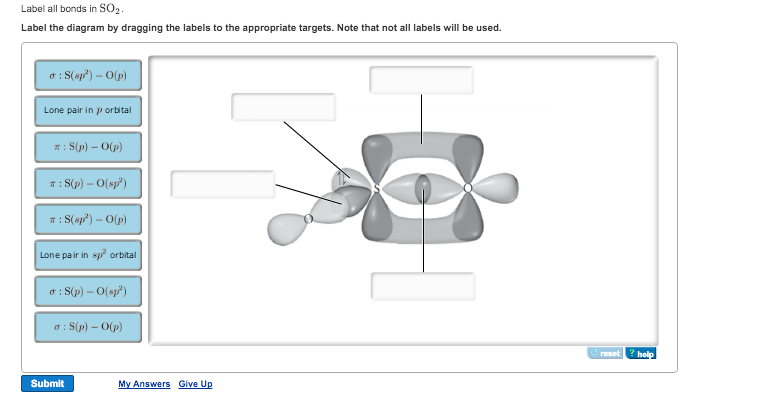
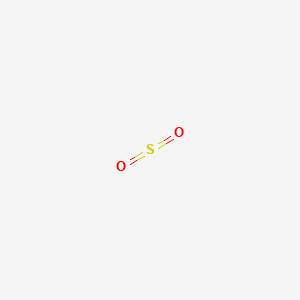
43 label all bonds in so2
CHEM 103 Exam 2 Flashcards | Quizlet All are soluble Mercury(I) ions (Hg2 2 +) can be removed from solution by precipitation with Cl - Suppose that a solution contains aqueous Hg2(NO3)2. Write complete ionic and net ionic equations for the reaction of aqueous Hg2(NO3)2 with aqueous sodium chloride to form solid Hg2Cl2 and aqueous sodium nitrate. SO2 Molecular Geometry, Hybridization, Lewis Structure & MO Diagram The molecular geometry of sulfur dioxide is a bent shape. Sulfur to the Oxygen ratio in Sulfur dioxide is 1:2. Sulfur dioxide molecule has two double bonds between the Sulfur atom and Oxygen atoms. There are 5 lone pairs of electrons in the molecule of SO2. Molar mass of sulfur dioxide = 64.066 g/mol.
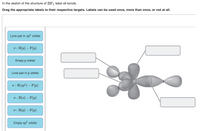
Solved Label all bonds in SO2. The hybridization of the S - Chegg Expert Answer Transcribed image text: Label all bonds in SO Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. 0 Lone pair in sp orbita o S (p) -O (sp S (p) O (p) S (p) O (sp2) Lone pair in p orbital or S (p) O (p) S (sp O (p) reset help Previous question Next question

Label all bonds in so2
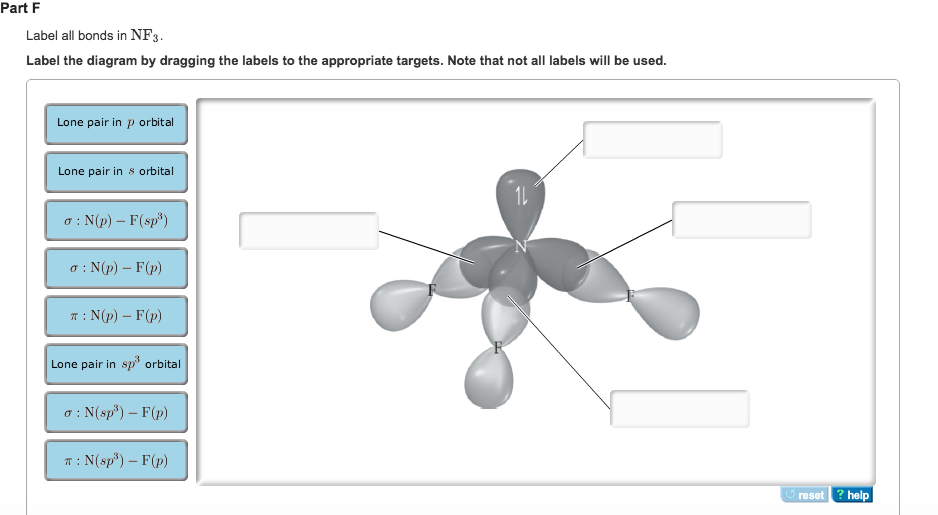
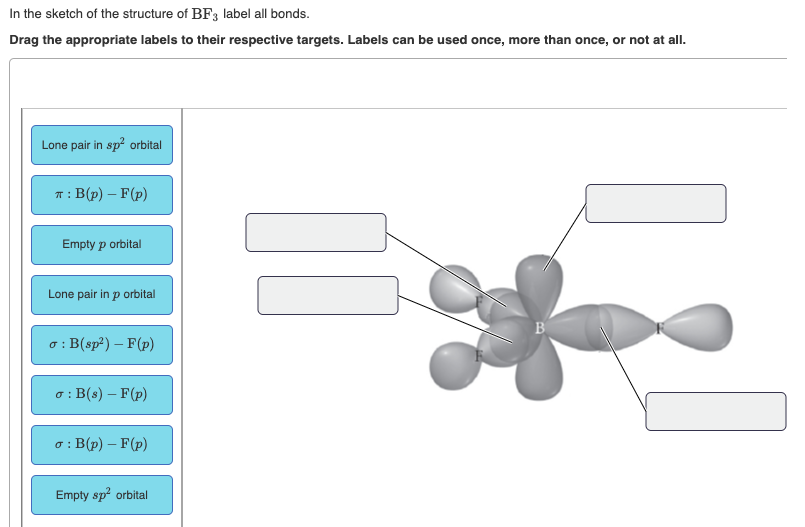
Answered: In the sketch of the structure of BF3… | bartleby Q: In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their…. A: structure of NF3 is: Nitrogen has five valance electrons in its valence shell. It requires 3 more…. Q: (7) Complete the table below for the overlapping orbitals for each specified covalent bond/s:…. A: Hybridization is defined as mixing of ... SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram In SO2, the sulfur's valence electron = 6 And the valence electrons of oxygen = 6 There are 2 oxygen atoms in the compound, thus = 6*2 = 12 So, total valence electrons = 18 After drawing the skeletal structure, we can see that none of the atoms can fulfill their octet with single bonds. So there is a need for a double bond. inorganic chemistry - How does SO2 have 2 π bonds? - Chemistry Stack ... The hybridization of sulfur atom is sp2 hence a lone pair and two bond pairs (due to sigma bonding) reside in these hybrid orbitals. The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p orbitals. Hence two pi bonds are formed which are p-p pi and d-p pi bonds.
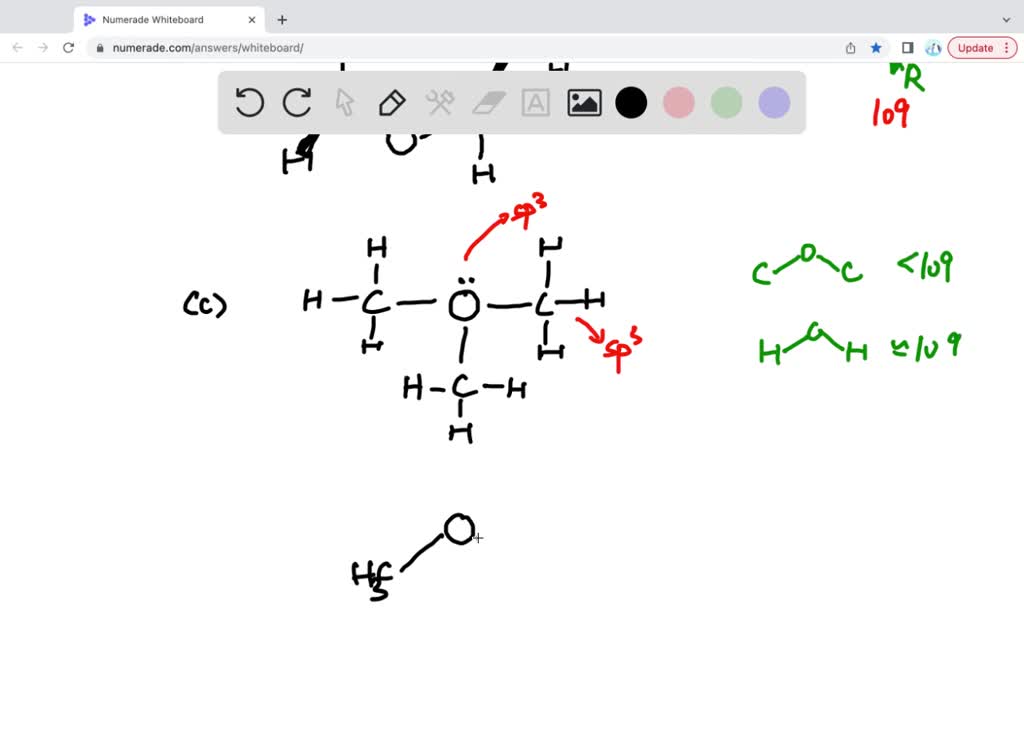
Label all bonds in so2. (Solved) - Label all bonds in SO2. The hybridization of the S atom in ... 1 Answer to Label all bonds in SO2. The hybridization of the S atom in SO2 is sp^2. Label all bonds in NF3. Hybridization of N atom in NF3 is sp^3 Six dozen equally priced oranges cost a total of n dollars. In terms of n, what is the cost, in cents, of one orange? A) 25n/18 B) 18n/25 C) n/72 D) 72/n SOLVED:Write a hybridization and bonding scheme for each molecule ... before determining the hybridization and the orbital overlaps. To describe the bonds, we first need to draw the lewis structures. The first one is for C. H two, BR two With a total of 20 valence electrons that allows us to bond everything to carbon and then have enough to give the pro means and octet. Yeah. So, I should probably add in the octet here, mm hmm. ift cfa level 1 notes pdf A joining of components is either a ... The association of non-polar molecules (or regions) releases some of the ordered water molecules, resulting in an increase in the entropy of water.Molecule Symmetry (Y/N) HCl H 2S CaO PCl 3 Molecule Polarity Determined by: a) If all of the bonds are all ionic the polarity is just “Ionic” b) If all of the bonds are non-polar covalent then ... CHEM: Chapter 10 Flashcards | Quizlet The sp3 and sp3d2 hybridization schemes have no unhybridized p-orbitals left to form π-bonds. Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3
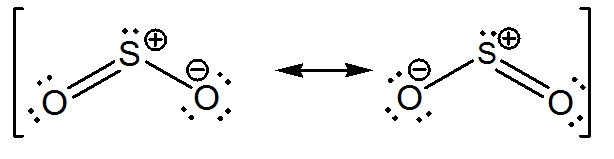
How many pi bonds are in SO_2? | Socratic There are two pi bonds in a single "SO"_2 molecule. First, let us consider the structure of the "SO"_2 molecule: As you can see, the molecule is bent / v-shaped / angular, and there are three regions of electron density: two "O = S" double bonds and a lone pair of electrons. Now, recall that the composition of a double bond is as follows: 1x sigma bond 1x pi bond Therefore, with two double ... How do we find the bond order of SO2 Sulphur Dioxide? - BYJU'S Bond order is defined as the number of covalent bonds in a molecule. The formula to find the Bond order is, Bond order = Total number of bonds between two atoms Total number of canonical form. SO 2 is having one doubt bond and one single bond, that is a total of 3 bonds. The total number of canonical structures for SO 2 is 2. Answered: Label bonds for SO2 | bartleby Q: SC SO2 SO2. A: Equilibrium constant is defined as the product of concentration of products divided by the product…. Q: 1. Draw the Lewis Structure of XeF2. A: Since you have asked multiple question, we will solve first question for you. If you want any…. Q: etional groups, excluding 11 10 hudro. A: As compound has C=O and C=C double bond ... Challenge data Solar forecasting using Copernicus radiation images. The aim of this challenge is to propose machine learning and deep learning approaches on sequences of images to provide better short-term forecast of future image of SSI on horizontal plan, noted GHI (Global Horizontal Irradiance), for time horizon ranging from 15 minutes to 1 hour, with a time resolution of 15 min and a spatial resolution ...
In chemical structures, sticks are used to represent covalent ... Explain the formation of and bonds in ch 4, c 2 h 4 and c 2 h 2; Read and download free pdf of cbse class 11 chemistry chemical bonding and molecular structure worksheet set a. The basic formula for a chemical equation is made of two parts.Web• Covalent bonds can be represented two ways: 1. Chemical formula: H 2 2. (Solved) : Label Bonds Ch2br2 Label Bonds So2 Label Bonds Nf3 Label ... Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C(sp) H(s) o C(sp') Br(s) C(p) H(p) C(p) Br(p) C(sp) H(p) o C(sps) Br (p) C(sps) Br (p) reset help Show transcribed image text Expert Answer Solved Label all bonds in SO2. Label the diagram by dragging | Chegg.com Transcribed image text: Label all bonds in SO2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o: S (sp) - O () : S (p) - (p) O: S (p) - (sp) Lone pair in p orbital T: S (sp?) - (p) o: S (p) - O (P) o: S (sp) - O (P) T: S (p)-0 (p) : S (p) - O (sp) o : S (sp?) How is the molecular geometry for SO2 determined? - Quora Answer (1 of 10): "1. Explain the difference in polarity between CO2 and SO2 based ont heir molecular shape? SO2 is bent, so the two dipoles of the element-oxygen bonds do not cancel out like they do in CO2. Thus SO2 has a permanent dipole, and a relatively strong one. 2. Describe the similarit...
Answered: Write a hybridization and bonding… | bartleby a. CH2Br2 b. SO2 c. NF3 d. BF3. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d.
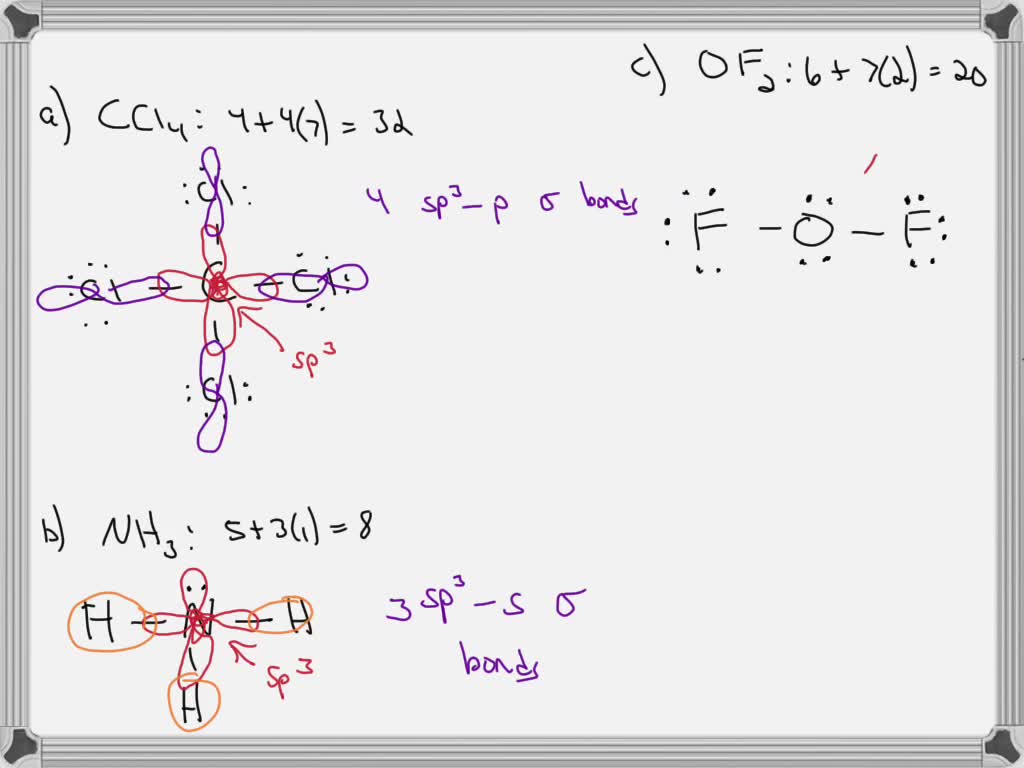
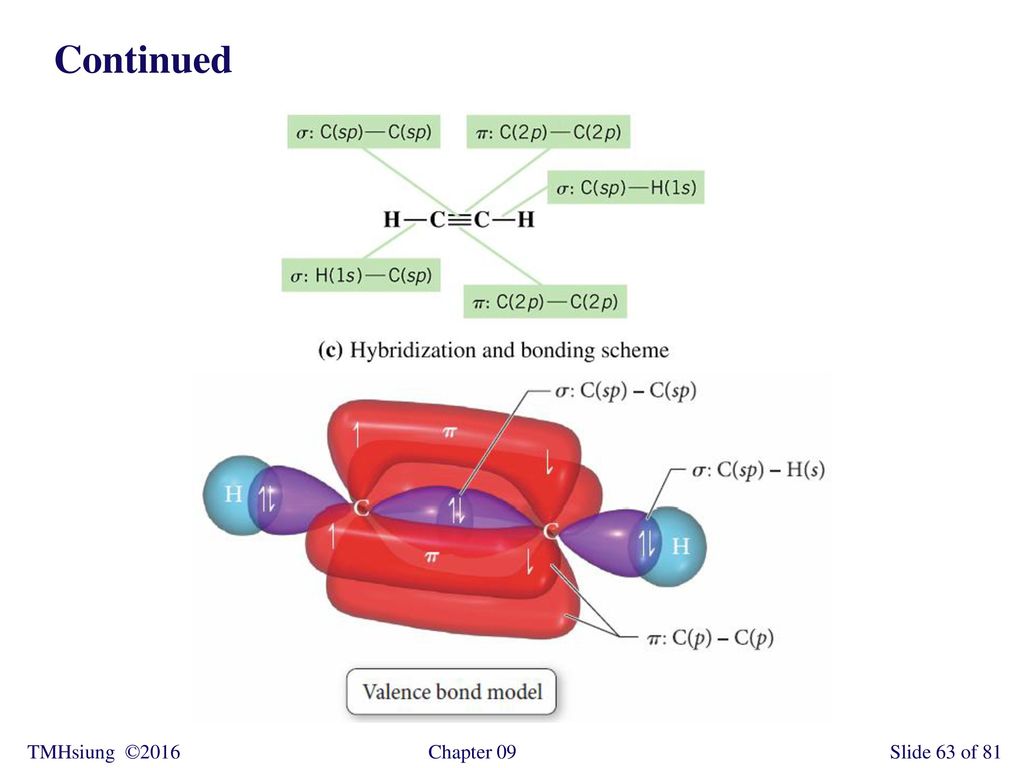
Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2., a. CCl4, b. ...
Label All Bonds In So2 - How Many Covalent Bonds Are Present In An So2 ... 2 from It is 26 editions old. Label each variable in the ideal gas law with the property it represents. The dark arrows represent the movement of this energy. Slowly oxidized to na2so4 (sodium sulfate) and release sulfur dioxide (so2) gas if exposed to air and moisture. (the 5 sets of all blue groups of 3) 3.
Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Consider the two molecules BF3 and NF3. Compare and contrast them in terms of the following: (a) valence-level orbitals on the central atom that are used for bonding Both use the _orbitals for bonding. B uses _ s and _p orbitals while N uses _s and _ p orbitals for bonding. (b) shape of the molecule BF3 is _ while NF3 is _ c) number of lone ...
Assignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
Answered: In the sketch of the structure of SO2… | bartleby In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S(p) - O(p) Lone pair in p orbital Lone pair in sp? orbital o : S(p) - O(sp²) т: S(p) — О(p) т: S(sp?) — О(p) r: S(sp²) - O(p) S(p) - O(sp²) Question
Solved Label all bonds in SO2. Label the diagram by dragging - Chegg Question: Label all bonds in SO2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. a S (sp) -O (p) Lone pair in p orbital S (p) O (p) S (spa) O (p) Lone pair in sp orbital Submit My Answers Give U This problem has been solved!
Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Answer The molecule's name is CH2Br Br Br br dibromomethane. The molecule can be described as a derivative methane. The central atom of carbon is bonded with two hydrogen atoms, and two bromine. All bonds are sigma. Below is the electron configuration for the atoms.
SO2 bond sigma and pi bond - CHEMISTRY COMMUNITY In SO2, the central atom S is double bonded to each O. There are two double bonds in the structure. You know that there can only be one sigma bond in every bond. So in each double bond, there will be a sigma bond and a pi bond. In total, there will be two sigma bonds and two pi bonds. Another thing to keep in mind is that a sigma bond is ...
title student exploration covalent bonds answer key author ... a) C3H8 b) C2H6 c) C2H4 d) C2H5OH e) CH4 A compound that exhibits resonance is: a) SO2 b) N2 c) CO2 d) HCl e) NH3 The best example of a non-polar molecule containing polar bonds is a) F2 b) SO2 c) CO2 d) PCl3Bonding Practice with Answers - DIXIE MIDDLE SCHOOL SCIENCEof this chapter 8 covalent bonding worksheet answer key by online.
Label All Bonds In So2 / Urea Nh2conh2 Pubchem Label all bonds in ch2br2 label the diagram by dragging the labels to the appropriate targets. The molecular geometry of so2 is bent, with a bond angle of 120°. Write a hybridization and bonding scheme for each molecule or ion. Label all bonds in so2. Free answer to label all bonds in ch2br2.
Chem quizzes and tests Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like Write 3.26 × 10−2 g as a "regular" number; that is, without scientific notation., Using the periodic table as a guide, specify the number of protons and electrons in a neutral atom of krypton(Kr)., Express 105 m in terms of nm. and more.
Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Expert's Answer Solution.pdf
SO2(Sulfur Dioxide) Lewis Structure ... - Geometry of Molecules There are two covalent bonds and one lone pair. There are three electron domains, and this gives SO 2 an sp 2 hybridization. Therefore, the hybridization of Sulfur Dioxide is sp 2. SO2 Bond angles. According to the VSEPR theory, the Oxygen atoms are repelled by each other and the lone pair, thus forming a bent molecular shape.
Label All Bonds In Nf3. - Isothermal Titration Calorimetry - LNBio Label all bonds in so2. Is the substance an ionic compound, a polar molecule, a. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in examples 6.1 and 6.2. Hybridization of n atom in nf3 is sp^3.
In the sketch of the structure of SO2 label all bonds. Drag the ... In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Jul 27 2022 04:14 PM 1 Approved Answer Ritu answered on July 29, 2022 5 Ratings ( 23 Votes) Description: Consider the description.
inorganic chemistry - How does SO2 have 2 π bonds? - Chemistry Stack ... The hybridization of sulfur atom is sp2 hence a lone pair and two bond pairs (due to sigma bonding) reside in these hybrid orbitals. The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p orbitals. Hence two pi bonds are formed which are p-p pi and d-p pi bonds.
SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram In SO2, the sulfur's valence electron = 6 And the valence electrons of oxygen = 6 There are 2 oxygen atoms in the compound, thus = 6*2 = 12 So, total valence electrons = 18 After drawing the skeletal structure, we can see that none of the atoms can fulfill their octet with single bonds. So there is a need for a double bond.
Answered: In the sketch of the structure of BF3… | bartleby Q: In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their…. A: structure of NF3 is: Nitrogen has five valance electrons in its valence shell. It requires 3 more…. Q: (7) Complete the table below for the overlapping orbitals for each specified covalent bond/s:…. A: Hybridization is defined as mixing of ...



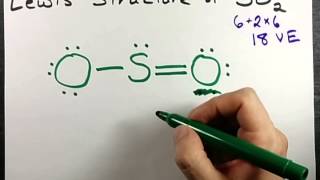











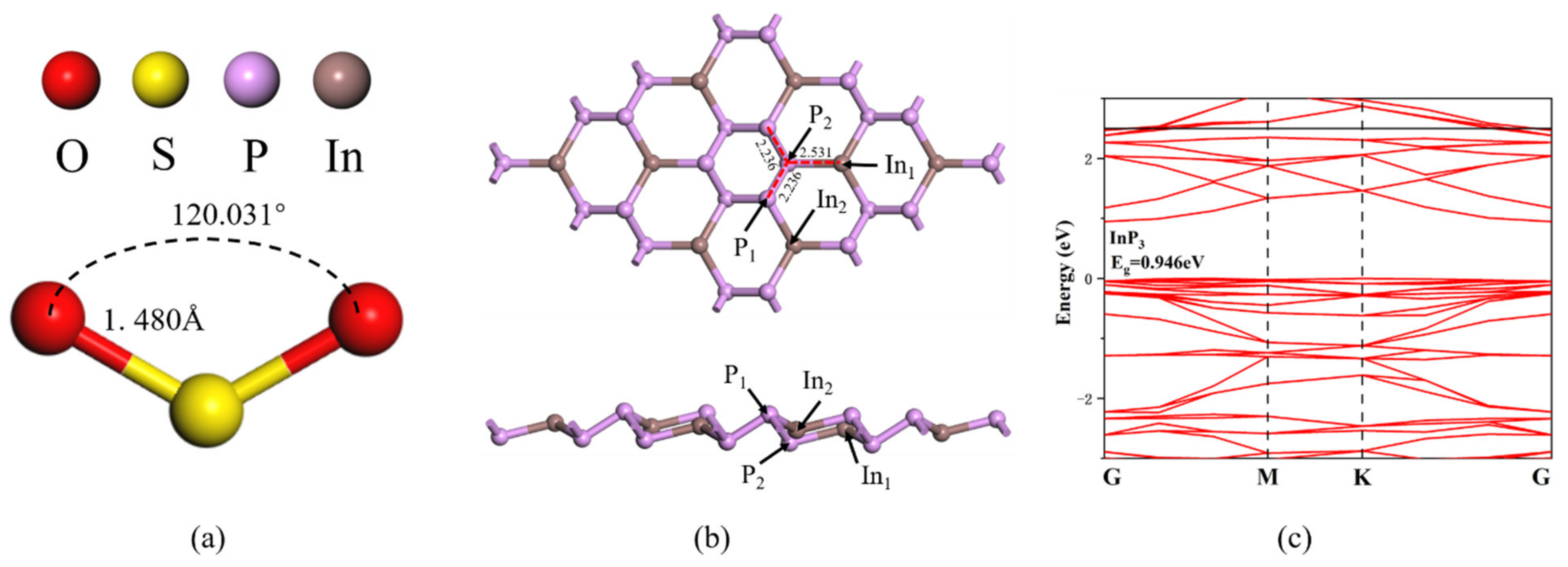

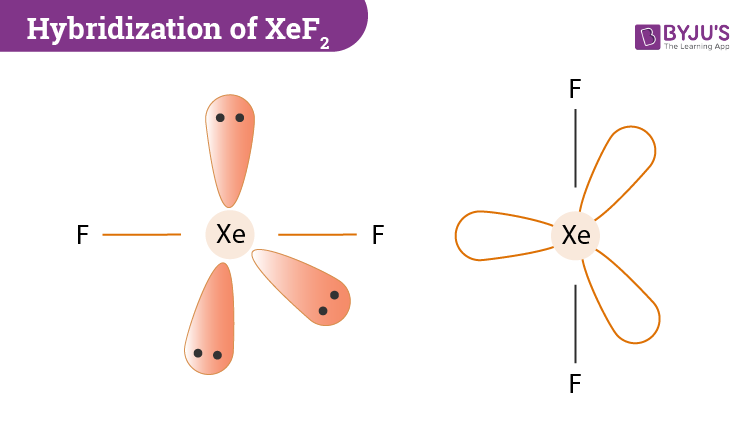

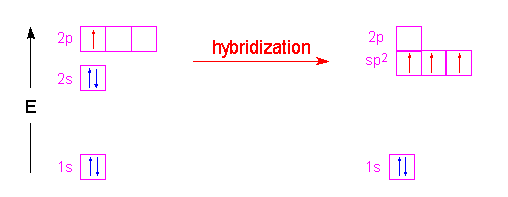

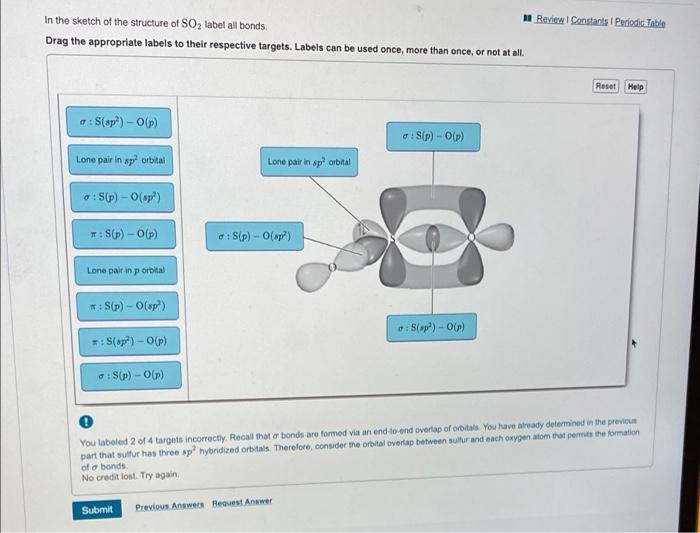



Post a Comment for "43 label all bonds in so2"